USP 800 Compliance expands on USP 797 and is intended to describe:
- The requirements and responsibilities for handling hazardous drugs
- Facility and engineered controls
- Procedures for deactivating, decontaminating and cleaning, spill control and documentation
USP 800 applies to health care personnel who handle hazardous drugs and all entities that transport, administer, store, prepare them. This often includes pharmacies, patient treatment clinics, hospitals and other health care institutions, veterinarians’ offices or physicians’ practice facilities.
One of the guidelines described by USP 800 is the sampling and analysis of hazardous drugs. So what is wipe sampling and why is it included in USP 800?
Wipe Sampling
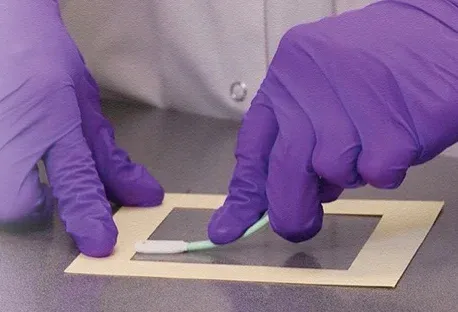
Wipe sampling is the act of wiping areas of interest with a swab which is later tested in a lab. The test can determine if certain hazardous drugs (HD's) are present and is designed to prevent contamination. This practice is designed to protect pharmacists, technicians, nurses, physicians, physician assistants, home healthcare workers, veterinarians, veterinary technicians, etc.
This form of sampling is extremely important when dealing with hazardous material because it protects patients and workers from exposure. According to USP 800, wipe sampling should be done a minimum of twice a year. It is not uncommon however for this to occur quarterly or more often as needed to ensure safety.
Critical Areas to Sample
USP 800 suggest the following areas as a minimum requirement:
- Interior of the C-PEC (Containment-Primary Engineering Control)
- Pass-through chambers
- Staging or work areas
- Floors and dispensing area near staging areas
- Outside HD buffer room or C-SCA
- Patient administration areas.
While this list broadly identifies the areas that need to be tested every 6 months, it is not intended to be an all-inclusive list. Hoods, carts, door handles, phones, and tabletops are also commonly tested just to name few.
What Drugs to Test For
This depends on the types of hazardous drugs being used in your facility. By testing for the hazardous drugs you will be able to readily identify contamination. USP-800 does however suggest testing for these common drugs:
- Cyclophosphamide
- Ifosfamide
- Methotrexate
- Fluorouracil
- Platinum Containing Drugs
If test results come back with measurable contamination, containment steps must be taken immediately. Once contained and disinfected, a second round of testing is advised.
Who Enforces USP 800 Compliance?
USP standards were created by The United States Pharmacudeial Convention along with the Fedral Drug Administration. The guidelines set forth are enforceable at the state level. This means states get to choose which standards to follow and also how those standards will be enforced.
Most commonly, states will embrace the USP 800 standards, but vary on how these standards are enforced. It is common for the State Board of Pharmacy to perform inspections in many states. In others the states public health department does inspections.
Stay Up To Date
Both USP-797 and USP-800 pharmaceutical regulations are helping healthcare practitioners by making compounding safer. All together, USP General Chapters are standards to assist practitioners in creating high-quality medicine and practices. They ensure patients are getting the right dosage, quality, and contaminant-free medication every time they go into their pharmacy.
With recent updates it is important to stay on top of regulatory guidelines in your area. The chief executive officer of USP says:
“From the time a medication is compounded, until the moment the patient takes it. We want to help ensure the patient benefit and reduce risks” Said Ronald T. Piervincenzi, Ph.D., chief executive officer, USP. “The updated USP standards will help ensure quality preparations for patients who rely on compounded medicines. While also minimizing the risk of exposure to hazardous drugs for healthcare personnel.”
The most recent update to USP 800 effect:
- Vented biological safety cabinet requirements
- Containment secondary engineering control (C-SEC)
- Control requirements
- Compliance under these regulations is critical for the safety of technicians and patients.
Hear To Help
Need help with wipe sampling? Give us a call at (712)-309-3680. At BalCon, we are available for consultation to help you through all stages, from design to occupancy.
For over 25 years, BalCon’s Lab Safety Services Team has been ensuring the safety of people, equipment, and lab environments. By exceeding all national standards, our Lab Safety group is the leader in comprehensive testing, certification and service.